UNIT 18 CHEMISTRY IN EVERYDAY LIFE
Syllabus
· Chemicals in Medicines and Healthcare
· Dyes
· Chemicals in Food
· Pheromones, Sex Attractants
· Detergents
· New High Performance materials
· Rocket Propellants
Chemistry plays a major role in all walks of our lives. Our daily needs of food, clothing, shelter, potable water, soaps, detergents, medicines etc are in one or the other manner connected with chemical compounds, processes and principles. Chemistry is the backbone of bulk of industrial materials like glass, cement, fertilisers , pesticides, paper, polymers, oils, fats, fuels etc., which are so very essential for the sustenance of our lives. Chemistry prefoundly influences the existence of human beings and the habitat.
CHEMICALS IN MEDICINES AND HEALTH CARE
In ancient practices of treatment of disease like Ayurvedic, the Unani systems or the modern allopatic system, the drugs used are chemical compounds of natural or synthetic origin. Some specific class of compounds are discussed below.
ANALGESICS
Analgesics are the drugs used for releaving pains in the body. Analgesics are of two types. These are narcotics and non-narcotics.
a. Narcotics : Drugs which produce sleep and unconciousness are called narcotics. Most of the narcotics are opium derivatives. Opium contains alkaloids such as codeine and morphine, which are highly effective analgesics. Morphine can be acetylated with acetic anhydride, to give heroin a very powerful analgesic. The biggest disadvantage with these are that they are habit forming i.e., one gets addicted to these narcotics.
b. Non-narcotics : These are not as effective as opium derivatives in relieving pain. But as these not habit forming, these are preferred over narcotics. Butazolidine, aspirin , novalgin etc are common examples of non-narcotic analgesics. Aspirin and analgin can also act as antipyretics. Analgesics are very commonly used these days particularly when there is fatigue resulting in body pains.
Antipyretics
Antipyretics may be defined as the chemical substance used to bring down body temperature in case of high fever. Adminstration of antipyretics often lead to perspiration , which bring down the body temperature . Common examples of antipyretics are aspirin, phenacetin and paracetamol.
Aspirin is one of the most commoly used antipyretics. It should not be taken in an empty stomach as it produces salicylic acid on hydrolysis inside the stomach. Salicylic acid so produced in empty stomach may ulcerate stomach wall and cause bleeding from there. Sodium or calcium salts of aspirin are more soluble and less harmful. However, paracetamol is comparatively safe.
TRANQUILLIZERS
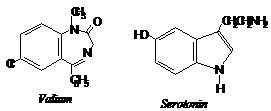
Tranquilizers are chemical compounds used for treatment of stress , mild and severe mental diseases. These are used to relieve stress , fatigue, by inducing sense of well being. They form an essential component of sleeping pills. Tranquilizers also form an important category of so called psychotherapeutic drugs. Derivatives of barbituric acid viz., veronal, amytal, nembutal, luminal and seconal are familiar drugs of this class. These derivatives are called barbiturates. Barbiturates are hypnotic, i.e., sleep producing agents. In addition to barbiturates, a large number of other nonhypnotic tranquiizers are known. Some important compounds of this class are discussed below.
Chlordiazepoxide and meprobamate are relatively mild tranquilizers suitable for relieving tension. Equanil is used in controling depression and hypertension.
ANTISEPTIC AND DISINFECTANTS
Antiseptics : The chemical substances which are used to kill or prevent the growth of micro-organisms are called antiseptics. These are not harmful to living tissues and can be easily applied on wounds , cuts , ulcers, diseased skin surfaces. These are also used to reduce odours resulting from bacterial decomposition of the body or in the mouth. They are therefore mixed with deodorants , face powders and breath purifiers.
Disinfectants : The chemical substances which are used to kill micro-organisms but they cannot be applied on living tissues are called disinfectants. Disinfectants play a major role in water treatment and in public health sanitation. These are commonly applied to inanimate objects such as floors, instruments etc.,
The same substance can act as disinfactant as well as antiseptics depending on its concentration. For example, a 0.2% solution of phenol acts as antiseptic and its 1% solution acts as disinfectant.
The common examples are :
(i) Chlorine is used for making water fit for drinking at a concentration of 0.2 to 0.4 ppm.
(ii) Dettol is an antiseptic. It is a mixture of chloroxylenol and terpeneol in a suitable solvent. Chloroxylenol has both antiseptic and disinfectant properties.
(iii) Bithional is antiseptic which is generally added to medicated soaps to reduce the odour produced by bacterial decomposition of organic matter on the skin.
(iv) Hexachlorophene is mainly used in soaps, creams , dusting powders and emulsions.
(v) Amyl metacresol (5-methyl-2-pentylphenol) is an antiseptic which is commonly used as a mouthwash or gargles in infections of mouth and throat.
(vi) A naturally occurring phenol derivative, thymol is used as a powerful disinfectant than phenol.
(vii) Iodine is a powerful antiseptic. It is used as a tincture of iodine which is 2 – 3 % iodine solution of alcohol - water.
(viii) Some organic dyes are also effective antiseptics. These are used for the treatment of infectious diseases. The common examples of antiseptic dyes are gention violet and methylene blue.
(ix) Idoform has been in use as an antiseptic powder of wound.
(x) Boric acid as a dilute aqueous solution is used as a weak antiseptic for eyes.
(xi) Hydrogen peroxide is used as non-irritating strong antiseptic.
ANTIMICROBIALS
Disease in man and animal may be caused by a variety of microorganisms e.g. bacteria, viruses etc. Microorganisms or microbes are extremely small organisms which can be seen only through a microscope. Any organism that causes disease is called pathogon. However, the body possesses an efficient natural defence mechanism, which operates at all times against potential pathogenic microbes. Intact skin is impervious to microbes. Many body secretions either kill microbes or inhibit their growth. Examples are lysozyme (a lipid splitting enzyme) in tears , nasal secretion and saliva, fatty acids and lactic acid in sweat and sebaceous secretions and hydrochloric acid in stomach. A breach in defence mechanism allows the pathogens to reach tissues and this causes infection. Invasion and multiplication of a organism in the infected host result in the onset of disease due to the destruction of the normal cell metabolism. In addition, toxins(toxic substances) produced by the microbes may adversely affect the tissues or organs of the host. The control of microbial dieases can be achieved in three ways :
(i) A drug which kills the organism in the body (bactericidal ),
(ii) A drug which inhibits or arrests the growth of the organism (bacteriostatic) and
(iii) Increasing immunity and resistance to infection of the body.
ANTIFERTILITY DRUGS
In highly populated countries, population control has assumed prime importance. One of the most covenient and widely used method of birth control is the use of oral contraceptives. Oral contraceptives belong to the class of natural products known as steroids. Steroids are the active ingredients of the pill functioning as an antifertility agent. It controls the female mensural cycle and ovulation. The birth control pill is essentially a mixture of synthetic estrogen and progestrone derivatives, which are more potent than the natural hormones. Some of the commonly used contain a combination of norethindrone and ethynylestradiol.
Mifepristone is a synthetic steroid that blocks the effect of progestrone and is used as ‘’morning after pill’’ in many countries.
ANTIHISTAMINES
These drugs are also known as anti-allergic drugs and are used to treat skin rashes. Since these allergic reactions are caused due to liberation of histamine in the body that is why these drugs are called antihistamines. Other than skin rashes these drugs are useful for conjunctvitis (inflation of conjunctiva of eye) and rhinitis (inflation of nasal mucosa). In sensational rhinitis and conjunctivitis, these drugs relieve sneezing , nasal discharge and itching of eyes, nose and throat. Common drugs of this group are diphenylhydramine, chloropheniramine and promethazine.


ANTIBIOTICS
Antibiotics are chemical substances produced by microorganism(bacteria, fungi and mould) that inhibit the growth or even destroy microorganisms. The development of synthetic methods has however , resulted in a modification of this definition. An antibiotic now refers to a substance (produced wholly or partly by chemical synthesis), which in low concentration inhibits the growth or destroys microorganisms by intervening in their metabolic processes. Antibiotic therapy has been likened to ‘setting one thief aganst another’. This is because antibiotics themselves are products of microbial growth. The first antibiotic , discovered by Alexander Fleming in 1929 from the mould Pencillium notatum, was pencillin. The general formula of pencillin is C9H11O4SN2R where R may be different for different members. For example,

With the substitution of different R groups, about six natural pencillin have been isolated so far. For example,
Pencillin | Nature of R |
Pencillin G or Benzyl pencillin | |
Pencillin F | CH3CH2CH=CH-CH2- |
Pencillin K | CH3(CH2)6- |
Ampicillin |  |
The antibiotics can be either bacterial or bacteriostatic.
Bactericidal Bacteriostatic
Pencillin Erythromycin
Aminoglycosides Tetracycline
Ofloxacin Chloramphenicol
Ampicillin and amoxycillin are semi-synthetic modifications of pencillin. It has become absolutely essential to test the patients for sensitivity (allergy) to pencillin before it is administered.

Pencillin has a narrow spectrum. These can be used for curing sore throat, gonorhoea, rheumatic fever, local infections, etc.
Streptomycin, neomycin etc are antibiotics that are used for the treatment of tuberculosis, meningitis, pneumonia, local infections , etc.
Broad spectrum antibiotics
These are the antibiotics which are effective against a variety of diseases. The common examples are tetracycline, chloromycetin and chloramphenicol which are effective against a variety of diseases. Therefore these can be used for curing typhoid, acute fever, dysentry, whooping cough , pneumonia, eye infections, certain urine infections. The structure of chloramphenicol is :

SULPHA DRUGS
These have great antibacterial powers and are used as medicines for various diseases. These are also antibiotics and protect the body against micro-organisms. These are used against diseases such as pneumonia , tuberculosis, diphtheria, etc. Some important sulpha drugs are sulphadiazine, sulphathiazole, sulphanilamide, sulphaguanidine, sulphaacetamide etc.

GERMICIDES
These are the chemical substances used to kill gems , fungi and virus. The common examples of germicides are phenol, cresols, formaldehyde , DDT, potassium permanganate solution (1%), chlorine, bleaching powder, hydrogen peroxide, etc.
ANTACIDS
Substances which remove the excess acid and raise the pH to apropriate level in stomach are called antacids. Acid Grastrics is one of the comonest ailments associated with digestion. It is caused by excess of hydrochloric acid in the gastric juice. Magnesium hydroxide, magnesium carbonate, magnesium trisilicate, aluminium hydroxide gel, sodium bicarbonate and aluminium phosphate are commonly used antacid. In recent years, omeprazole and lansoprazole are also marketed as antacids. These prevent formation of acid in stomach.

ANAESTHETICS
These are chemical substances which produce general or local insensibility to pains and other sensations. Cocaine, novocaine are local anaesthetics. Chloroform , diethyl and vinyl ethers , etc are general anaesthetics.
DYES
Dyes are chemical substances used to impart colours to textiles, foodstuffs, silk, wool and other objects. All coloured substances are, however , not dyes. A substance can act as a dye if it satisfies the following conditions :
(i) It must have a suitable colour.
(ii) It must be able to fix itself permanantly to the material being dyed.
(iii) When fixed, it must be fast to light and washing.
(iv) It must be resistant to the action of water, soap, acids and alkalies or other solvents used in dry cleaning etc.
A dye is an organic compound which can absorb light in the visible region of the electromagnetic spectrum (400 nm to 750 nm). The part of the light which is reflected back gives the colour of the dye and this colour is complimentary to the colour of the light absorbed. Therefore, the colour of a particular dye depends upon the nature of wavelength absorbed and released in the visible region. For example, if a dye absorbs in the visiblle region corresponding to green colour, then it will appear as violet, which is the complimentary colour of green. Similarly, if a dye absorbs blue colour, it will appear yellow which is complimentary colour of blue. Thus, the dyes impart colour to fabric by absorbing the complimentary colour.
Classification of Dyes
Dyes can be broadly classified into two types on the basis of :
(i) their chemical constitution.
(ii) Their applications.
Classification of dyes on the basis of chemical constitution
On the basis of the characteristic structural units constituting the dyes, these may be classified as :
1. Indigoid dyes : These contain the indigoid group. For example, indigo.

2. Azo dyes : These dyes contain the azo group ( - N = N -). For example, congo red, Orange

Some other examples of azo dyes are :




Among these , orange-I , methyl orange and methyl red are acidic dyes, while aniline yellow , butter yellow are basic dyes.
3. Phthalein dyes
These dyes contain the phthalein group. For example, phenolphthalein.

4. Triphenyl methane dyes
These dyes contain the triphenyl methane group as shown below. For example, malachite green.

5. Anthraquinone dyes
These contain anthraquinone group. For example, alzarin.

6. Nitro dyes
These contain nitro group. For example, martius yellow dye.

Classification of dyes on the basis of their applications
The dyes are classified into the following types on the basis of their applications.
1. Acid dyes : These are usualy sodium salts of sulphonic acid (-SO3H) or carboxylic acid (-COOH) . The dye can be applied to wool , silk, nylon and cannot be used to dye cotton.
The common examples are orange-I , orange-II , congo red, methyl red etc. The dye orange-I is prepared by coupling of diazotised sulphanilic acid with a-naphthol.

2. Basic dyes : Basic dyes contain amino groups which in acid, form water soluble salts. These dyes get attached to the anionic sites present on the fabrics. Such dyes are used to dye reinforced nylons and polyesters. Aniline yellow and malachite green belong to this class of dyes.
3. Direct dyes : These are water soluble dyes. As the name suggests, these dyes are directly applied to the fabric from aqueous solutions and are practically suited for fabrics like cotton, rayon, wool, silk and nylon which form hydrogen bonds with water. Martius yellow and congo red are important examples of this class of dyes.
4. Disperse dyes : These dyes in the form of minute particles of a suspension diffuse into the fabric. Such dyes are used for dyeing synthetic fibres like polyesters, nylon and polyacrylonitrile. Many anthraquinone disperse dyes are suitable for application to synthetic polyamide fibres.
5. Fibre reactive dyes: These dyes attach themselves to the fibre by an irreversible chemical reaction. The dyeing is fast and colour is retained for a long time. The bonding is through the substitution of leaving group of dye via the hydroxy or amino group of fibres (cotton, wool or silk).
6. Insoluble azo dye : These dyes are obtained by coupling phenols , naphthols , arylamines, aminonaphthols adsorbed on the surface of a fibre with diazonium salt. Cellulose, silk, polyester, nylon, polypropylene, polyurethanes, polyacrylonitriles and leather can be dyed by using these type of dyes. Azo dyes also find use in food stuffs, cosmetics, drugs, biological stains such as indicators in chemical analysis. Use of such dyes for colouring food stuffs is not permitted now. Their use is now decline in other areas also.
7. Vat dyes : Vat dyes are insoluble in water and cannot be used directly for dyeing. However, on reduction to a leuco form they become soluble in presence of an alkali and acquire affinity for cellulose fibres. A solution of leuco form can be applied for deying or printing. On reoxidation (usually in presence of air) the original insoluble dye is formed within the structure of the fibre. Indigo and indgosol O are versatile dyes which belong to this class.

Indigosol O is readily soluble in water, has an affinity for cellulose and can be rapily and quantitatively oxidised on the fibre with the formation of indigo. It is especially suitable for wool.
8. Mordant dyes : These dyes are primarily used for dyeing of wool in presence of metal ions. The metal ion binds to the fabric and the dye acting as a ligand coordinates the metal ion. The same dye in the presence of different metal ions imparts different colours to the fabrics. Alizarin imparts rose red , blue, brownish red, violet and red colour to the fabric in the presence of Al3+, Ba2+, Cr3+, Mg2+ and Sr2+ ions respectively.
COSMETICS
The word cosmetics is derived from the Greek word Kosmetikos. It means decorating, butyfying or improving the complexion of skin. In India
1. Creams : There are many types of face creams and the most common being cold cream, vanishing cream, cleansing cream and bleaching cream. All types of face creams , except vanishing creams contain the same types of ingredients.
(i) Oils such as almound oil, olive oil and mineral oil.
(ii) Fats and waxes such as bees wax, lanolin (from sheeps wool), paraffin, petroleum , sermaceti(from the head of sperm whales).
(iii) Water
(iv) An emulsifier.
(v) A perfume or a blende of perfume.
The nature and amount of ingredients used depend upon the type of the cream. For example, a cold cream and a cleansing cream may have same components but a cleansing cream will contain less water and more of oils than the cold cream. A large mineral oil content improves the cleansing efficiency of creams and tends to stiffen the cream. Though water lowers the cleansing efficiency of cream, yet it has sofening effect and makes the creams finer and more lustrous. Bleaching creams are similar to cold creams but they contain added bleaching agents like hydrogen peroxide, mercuric chloride or lactic acid.
Vanishing creams differ from the other creams because they contain a humectant like glycerine but no oils. In place of bees wax and other waxes, these contain stearic acid. Common components of these creams are :
(a) Cold creams : These contain bees wax, paraffin oil, borax, water and perfume oil. Mineral oils used are generally the light and medium viscosity grades which do not produce a viscous film on the skin. These have excellent cleansing properties.
(b) Vanishing creams : These contain stearic acid, alkali, water and humectant. Alkali forms soaps with part of stearic acid, the soap which emulsifies the fatty acids in water. To get creams of good consistency, having good texture, pure caustic potash is usually used as alkali. Caustic potash alone will form too soft creams, while caustic soda alone form hard creams. The most commonly used humectant is glycerine. It enhances pearliness and helps to prevent crust on the surface. It also renders the film deposited on the skin surface less dry to touch and sufficiently tacky to retain a thin film of the face powder superimposed on it.
Generally, some additional ingredients such as perfumes , preservatives, emollients and auxilliary emulsifying agents such as glyceryl monostearate are added. Some cleansing creams also contain antiseptics. The most commonly used antiseptics in cosmetics is hexachlorophene. Certain emollients are polyhydric alcohols such as propylene glycol, glycerol, sorbitol etc.
2. Perfumes
Perfumes are the materials, used to provide fragrance. Several requirements have to be fullfilled to make a good perfume and any material, which just gives good smell, may not be a perfume.
A perfume invariably consists of three ingredients : a vehicle, fixatve and odour producing substance.
Vehicle : The vehicle is also called solvent. The role of solvent is to the keep the odour producing substances in solution. Ethanol and water mixture is the most common vehicle used in perfumery.
Fixative : The function of the fixative is to equalise the rate of evaporation of various odouriferous compoents of the perfume by suitably adjusting their volatility. Sandalwood finds use as fixative. Other substances used as fixative are benzoin, glyceryl diacetate and esters of cinnamic alcohol.
Odourous substances : Both natural and synthetic substances are used to impart odour to a perfume. For example, terpenoids like linalool which occur in essential oils are natural odour producing compounds, while anisaldehyde (p-methoxybenzaldehyde) is a synthetic odour producing compound.
TALCUM POWDER
Talcum powder is used to reduce irritation of the skin. Talcum powders like face powders contain talc (Mg3(OH)2Si4O10). Chalk, zinc oxide, zinc stearate and a suitable perfume act as the other main constituents of talcum owder. Often specific ingredients like antiseptic and cooling agents are added. The role of talc is to act as a powder base and to make skin smooth. Chalk absorbs secretion (perspiration) without showing any evidence of such absorption. Zinc oxide masks enlarged pores and minor blemishes, whereas zinc stearate makes powder adhere to the skin. Baby talcum powders contain considerable amounts of zinc stearate for adhesiveness and boric acid , for antiseptic purposes. Talcum powders need to dusted with care to prevent inhalation of the fine paricles , which irritate the lungs.
DEODORANTS
Deoddorants are applied primarly to mask the body odour. The body odour results from the bacterial action following perspiration. A deodorant must therefore , possess anti-bacterial properties. Aluminium salts, have been found to possess excellent antibacterial properties. In addition to aluminium salts, ZnO also find use in deodorant preparations because they are astringents as well as antiseptics. Phenolic antibacterials, which have been figured as effective body deodorant are parachlorometaxylenol and dichlorometaxylenol having following structures.

Powder formulations generally have deodorants.
CHEMICALS IN FOOD
Many chemicals are added to food for their preservation and enhancing their appeal. These include flavourings, sweetners, dyes, antioxidants, fortifiers, emulsifiers and antiforming agents. With the exception of the preservatives, fortifying agents, antioxidants and artificial sweetners, the remaining classes of chemicals mentioned above are added either for ease in processing or for cosmetic purposes, in the real sense these have no nutritive value.
Antioxidants
All the foods which contain substances like saturated oils and fats undergo deterioration on storage because of atmospheric oxygen. To prevent their spoilage, certain chemical substances are added which prevent their oxidation. These are called antioxidants. Thus, antioxidants are chemical substances which prevent oxidation and subsequent spoilage of the food.
The common antioxidants are butylated p-hydroxyanisole (BHA) or butylated p-hydroxytoluene (BHT), or several esters of gallic acid ( such as propyl galate, dodecyl gallate) and lecithin.


The addition of BHA to butter increases its storage life from months to years. Some times BHT and BHA are added in combination with citric acid or ascorbic acids to produce a more active synergietic effect. Sulphur
Artificial sweetners
The artificial sweetners are food additives. The first popular artificial sweetner was saccharin. It was marketed as its water soluble sodium or calcium salt . Saccharin is approximately 360 times more sweeter than cane sugar. It has proved to be a lifesaver for countless diabeics and is of great value to people need to control intake of calories.
Besides saccharin, the other commonly marketed artificial sweetners are given below :




Aspartamin is unstable at cooking temperatures, limitting its use as a sugar substitute to cold foods and soft drinks. Alitame is more stable than aspartame during cooking. One potential problem with alitame and similar types of high –potency sweetners is the difficulty in controlling sweetness of food. Sucralose is predicted to become a great commercial success.
Relative sweetness of some of carbohydrates and other compounds | |
Fructose | 173 |
Invert sugar | 123 |
Sucrose | 100 |
Glucose | 74 |
Xylose | 40 |
Galactose | 32 |
Maltose | 32 |
Lactose | 16 |
Nectarin | 50,000 |
Saccharin | 36000 |
Cyclamate | 7,100 |
Aspartame | 18000 |
Sucralose | 65000 |
Alitame | 200000 |
Prservatives
These are the chemical substances which are added to the food materials to prevent their spoilage and to retain their nutritive value for long periods. These preservatives prevent the rancidity of food and inhibit the growth or kill the microorganisms.
The common salt, sugar , oils and spices provide a medium that resists the activity of micro-organisms in food. The preservation of food by adding sufficient amount of salt to it is called salting. It is used for the presevation of raw mango, amala, beans , tamirind, fish , meat etc. Sugar syrup is used for preserving many fruits such as apples, mango , strawberry, carrot etc. Besides sugar and salts other substances such as vinegar, oils, spices, citric acid are also used as food preservatives, which are used for pickles, ketchups, jams, squashes etc.
The growth of micro-organisms in food materials can also be prevented by adding certain chemical substances such as benzoic acid in the form of its sodium salt (sodium benzoate), potassium metabisulphite (source of sulphurdioxide) , sorbic acid, calcium propionate etc. Certain food preservatives such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) for eddible oils also act as antioxidants.
Edible colours
These are the chemical substances which are used for imparting colour to the food and increase the eye appeal and compliment of a definite flavour. The main condition for using a colour in food is that it should be harmless to human health. It should be stable towards the action of acids , alkalies, high temperature , day light and the long storage. The colouring agents which are commonly used for foods are of natural and synthetic origin. Many compounds contain coloured compounds (pigments) which may be extracted and used as colouring agents e.g. chlorophyll , b-carotenes, alizarin, indigo etc. The common natural colours are chlorophyll ( green colouring matter extracted from leaves) saffron (prepared from flowers) turmeric ( dried and and ground root of ginger) , caramal (obtained by heating sugar) etc.
The aniline dyes which are superior to the natural dyes in reliability , purity and brightness of colour are replacing natural colours. For example, amaranth is a synthetic azo dye. It is reddish brown with a saline taste. In it dissolves in water giving magenta red colour.

Other synthetic colours used in food products are indigo carmine (blue) , tartrazine (yellow) , erythrosin, carmoisine (fast red) , ponceau (fast green), sunset yellow, etc. Some combination of these colours are useful to obtain different colour or shade of a particular colour , suitable for various food products. The use of synthetic colours is to follow the latest rules and regulations regarding the permissibility of any colour to food.
PHEROMONES AND SEX ATTRACTANTS
Insects communicate with each other and with their environment by means of organic chemicals secreted in very minute amounts. These substances are called pheromones (Greek pherin to transfer hormone to excite). Thus chemical substances excreted by one individual of a species that brings forth a response to another individual of the same species is called pheromone.
Insect pheromones are generally classified by the response they elicit. These are :
(i) Alarm pheromones which spread alam.
(ii) Sex attactants which help the different sexes of the same species to find one another.
For example, a pheromone secreted by one bee helps to alert other bees to the location of food source. You have seen a line of ants, all following the same individual trail across a floor. This is because one ant has found a food source and has laid down a chemical , a trail of pheromone on its return to the nest. Other ants then follow this chemical trail back to the food source. Insects also use pheromones exclusively to attract members of opposite sex for mating.
The insects are of very small size and therefore they excrete only minute amounts of a pheromone. However, these compounds are active in extremely small amounts. For example, a typical female sex insect can carry 10-8 g of sex attractant , yet it is sufficient to attract over a milion males from miles away. A male gypsy moth can smell a female at a distance of 7 miles.
The structures of all the pheromones are very complex. For example , geraniol and citral are recruting pheromones for honey bees while isoamyl acetate is a bee alam pheromone.

The folowing compounds have sex attractant activities for different species of insects.

Sex attractant pheromones have been used for pest control. In this method, a large number of traps , baited with small amount of the sex attractants of the female insects are used to trap males so that the breeding of the insect is decreased. In another method, male insects may be lured by the sex attractant pheromone, trapped and used to monitor their population. This helps to find the best time for applying pesticides by monitoring these traps.
DETERGENTS
As a result of high dissolving power, naturally occuring water always contains dissolved materials particularly ionic substances. Hard water contains certain metal ions, such as Ca2+ and Mg2+. These ions react with soap(sodium salts of stearic acid and similar organic acids), to produce curdy precipitate of calcium and magnesium soaps. This precipitate adheres to the clothing and blocks the ability of soaps to remove oil and grease from fabrics. Synthetic detergents are very similar to the salts of fatty acids found in soap, except that they are manufactured chemically from materials other than animal fats. Examples include salts called sodium alkylbenzenesulphonates, which have the general structure,

Their advantage over natural soaps is that they work in hard water. The anions of synthetic detergents do not precipitate in the presence of Ca2+ or Mg2+ ions, so their cleansing action is not affected by hard water.
Types of detergents
Detergents are mainly classified into three categories, namely anionic, cationic and non-ionic. Long chain alcohols are used in the manufacture of some of the synthetic anionic detergents. The long chain alcohols are treated with concentrated sulphuric acid to form alkyl hydrogen sulphates of high molecular mass and finally the alkyl sulphates are neutralised with alkali to form salts.

A detergent of the above type is an anionic detergent , named so as a large part of the molecule is an anion. The single anionic detergent in largest use today is alkylbenzene sulphonate.
The anionic detergents are also effective in slightly acidic solutions to form an alkyl hydrogen sulphate which is a soluble material, whereas the soaps react with the acidic solutions to form insoluble fatty acids.
A second type of detergents is cataionic detergents. These are mostly acetates or chlorides of quaternary amines. Being more expensive than the anionic detergents they find limited use. Such detergents however, possess germicidal properties and are used quite extensively as germicides. Cetyltrimethylammonium chloride is an example.

Some of the detergents are nonionic, like the esters of high molecular mass formed by reactions between polyethylene glycol and stearic acid.

Some liquid dishwashing detergents are of nonionic type.
In the past many detergents , caused concern for causing pollution in rivers and waterways. Hydrocarbons used earlier for manufacture of detergents had a great deal of branching in the hydrocarbon tail as shown below. This caused pollution.

Detergent molecules associated with branched hydrocarbon tail which is a source of pollution.
The hydrocarbon side chain stops bacteria from attacking and breaking the chains. This results in slow degradation of detergent molecules leading to their accumulation. These days the amount of branching can be kept to a minimum. Unbranched chains are more prone to attack by bacteria so the detergents are more easily biodegraded and pollution is prevented.
NEW HIGH PERFORMANCE MATERIALS
1. Carbon fibres
Carbon fibres are a new breed of high performance materials, which have attracted world-wide attention and hold great promise for the future. This is because of the fact that these fibres are stronger than steel, stiffer than titanium and lighter than aluminium. These qualities have placed carbon fibres on the top list of many novel materials available today.
Carbon fibres are produced in a number of ways and form a variety of starting materials or precursors such as viscose rayon, polyacrylonitrile, pitch, resins, gases such as methane and benzene.
They are obtained by heating regenerated fibres (such as rayon) or synthetic fibre (nylon or polyester0 in absence of oxygen. As a result of heating the fibres decompose to form carbon fibres.
Their characteristics are strongly influenced by the manufacturing techniques employed.
Carbon fibres reinforced in a light weight matrix, generally an epoxy resin, polyester resin or polyamide, are called Carbon Fibre Reinforced Plastics (CFRP) . When the carbon fires are reinforced in a carbon matrix, they are known as Carbon Fibre Reinforced Carbon (CFRC) , commonly known as carbon-carbon composites.
On the basis of the characteristics, carbon fibres , carbon fibre reinforced plastics(CFRP) and carbon fibre reinforced carbons (CFRC), their applications can be broadly classified into three categories :
1. High technology sector including aerospace, military and nuclear fields.
2. General engineering sector including sports, transportation and chemical fields.
3. Biomedical sector.
In the aerospace sector, the components are used for aircraft wings, tail parts, helicopter blades, and wing spoilers. The floor decking of air ships is also made from carbon fibre-reinforced composites. Interest in applications involving helicopters continues and it is believed that the first all-composite aircraft to fly will be a helicopter. Helicopter rotor blades made from CFRP not only give better performance but also are less expensive than the metal blades.
Carbon fibre in the form of carbon fibre-reinforced carbon commonly known as carbon-carbon composites, find in many facinating applications in space. An unusual application of carbon-carbon composites is its use in brakes in heavy and fast jet aircrafts. Carbon-carbon composite brakes perform three to five times better than their steel counterparts.
The high thermal conductivity of carbon fibres enhances the heat dissipation in components such as wall material of nuclear fission reactor, gears, brake pads, bearings, fan blades, automobile parts and other friction related products, further the low coefficient of thermal expansion makes it possible to design structures with zero or verly low planar thermal expansion.
Carbon fibres in the form of CFRP find many uses in the area of sports-goods. Very superior specific strength and stiffness , coupled with good fatigue resistance , make them versatile materials for fishing rods, sky poles, tennis and badminton rackets, racing cycle frames and racing car bodies.
In biomedical field, carbon fibres have exciting applications, such as components of bone plates, hip joint prostheses, ligaments and hydraulic motors for artificial heart implants. Activated carbon fibres are finding increasing applications in water treatment, gas masks , air filters, catalyst carriers for platinum and so on. Activated carbon fibres in textile form are used in extremely hostile environments. The main advantages using carbon fibres are that they can be woven in any form and a surface area of as high as 3000 m2/g can be obtained.
Carbon fibres in India are used in defence sector as nose tips and head shields of missiles (like ‘Agni’) by DRDO , Hyderabad
CERAMICS
The term ceramics come from the Greek word keramikos which means burnt stuff, indicating thereby, that desirable properties of these materials are normally achieved through a high temperature heat treatment process called firing. In the past , the most important materials in this class were the traditional ceramics, prepared from clay , (kaoloinite) a silicate. In the category of traditional ceramics we have porcelain , bricks, tiles, glass and temperature resistant ceramics. Significat progress has been made in understanding the fundamental characteristics of these materials and consequently a new geneation of these material has come into existence. The term ceramics has taken on a much broader meaning.
Most ceramics materials fall into an application – classification scheme which is given below.
Clay Products : Porcelain , pottery, tablewares, sanitary fittings, building bricks, tiles and sewer pipes.
Glass Ceramics : Kitchenware
Refractory materials : Refractory bricks used as furnace linings.
Abrasive Ceramics : Cutting and grinding tools ( Examples silicon carbide and tungsten carbides)
Recently a family of ceramics have been found to be superconductors with high critical temperatures. One such material is yttrium baium copper oxide, which has a critical temperature of about 92 K. New superconducting ceramic materials reported to have even higher critical temperatures have been and are currently being developed. Several of these materials and their critical temperatures are listed below.
Superconducting Ceramic Materials and their critical temperatures.
Material | Elements present in the material | Critical temperature (K) |
YbBa2Cu3O7 | Y, Ba, Cu, O | 92 |
Bi2Cr2Ca2Cu3O10 | Bi, Sr, Ca, Cu, O | 110 |
Tl2Ba2Ca2Cu3O10 | Tl, Ba, Ca, Cu, O | 125 |
HgBa2Ca2Cu2O8 | Hg, Ba, Ca, Cu, O | 153 |
The technological potential of these materials is extremely promising as their critical temperatures are above 77 K. Numerous applications of super conducting materials exist. Some of these are :
(i) Electrical power transmission.
(ii) Magnets for high-enrgy acclerators.
(iii) High-speed switching and signal transmission for computer.
(iv) High speed magnetically levitated trains (trains which move on air without rails).
MICROALLOYS
24 karat gold is traditionally not used to manufacture jewellary as it cannot withstand everyday wear. To overcome this difficulty, recently a number of microalloyed high karat gold qualities have been marketed with gold content of 99.95 % or even higher. These microalloys are found to possess improved hardness and strength. This makes it possible for jewellers to use in the trade 24 karat hall mark and at the same time to retain its finish and structure in everyday use. In some of the cases the microalloyed gold is found to show physical properties very similar to platinum.
Steel and steel alloys are very familiar. Now microalloyed steels have started engaing the attention of researchers. Microalloyed steels posses excellent combinations of strength and toughness , formability and weldability. The usual microalloy steels contain niobium, titanium and vanadium. In some cases microalloyed materials have shown excellent resistance to deformation. These materials have been found to retain their shape even under very high loads.
ROCKET PROPELLANTS
Many satellites have been launched by diferent countries for space reasearch. India
Depending upon their physical state, propellants are classified into the following types :
(i) Solid propellants
(ii) Liquid propellants
(iii) Hybrid propellants.
1. Sold propellants : The solid propellants are mixtures of solid fuel and a solid oxidiser. They are further divided into two classes.
(a) Composite propellants : These are solid propellants which use polymeric binder such as polyurethane or polybutadiene as a fuel and a solid oxidiser such as ammonium perchlorate, nitrate or chlorate. The performance of these propellants can be increased by using some additives such as finely divided magnesium or aluminium metal along with the fuel.
(b) Double base propelants : These are solid propellants which mainly use nitroglycerine and nitrocellulose. The nitrocellulose gels in nitroglycerine set in as a solid mass.
The main disadvantage of solid propellants is that these propellants once ignited will continue burning with predetermined rate and do not have the start and stop capability.
2. Liquid propellants : Liquid propellants consist of a combination of an oxidizer such as liquid oxygen, nitrogen teroxide (N2O4) or nitric acid and a fuel such as kerosene, alcohol, hydrazine or liquid hydrogen . These are further classified as :
(a) Monopropellants : The propellants in which a single chemical compound acts as a fuel as well as an oxidizer are called monopropellants. For example hydrazine, nitromethane, methyl nitrate, hydrogen peroxide etc. Except hydrazine, the other compounds contain both the oxidizer and the fuel elements in the same molecule.
(b) Bipropellants : These are propelants in which the fuel and oxidiser are stored separately but are allowed to combine at the time of combustion. For example, kerosene and liquid oxygen.
Liquid propellant systems are also classified as either storable or cryogenic. The crogenic systems generally show high performance.
Advantages of Biliquid Propellants over Solid Propellants
(i) The biliquid propellants give higher thrust than solid propellants.
(ii) The thrust generated by liquid propellants can be controlled by switching on and off the flow of propellants. On the other hand , thrust cannot be controlled in solid propellants.
3. Hybrid propellants : These are the ropellants which consist of solid fuel and a liquid oxidiser. Example, liquid N2O4 (liquid oxidizer) and acrylic rubber (solid fuel).
Examples of Propellants used in different rockets
(i) Saturn booster rocket of American space programme used a mixture of kerosene and liquid oxygen as the propellant in the initial stage whereas liquid oxygen and liquid hydrogen were used as propellant in high altitudes.
(ii) Russiaan rockets such as Proton used a liquid propellant conisting of kerosene and liquid oxygen.
(iii) The Indian rocket SLV-3 and ASLV used composite solid propellants.
(iv) The Polar Satellite Launch Vehicle (PSLV) is a remote sensing satelite. India India
QUESTIONS
1. How are antiseptics distinguished from disinfectants ? Give two examples of each pof the substances.
2. What is an antiseptic ? Give the name of the first antibiotic discovered ?
3. List two major classes of antibiotics with an example of each class.
4. What are antacids ? List some of the compounds , which are used as atacids .
5. Describe the following with suitable examples.
(i) Tranquilizers (ii) Antifertility drugs (iii) Antihistamines.
6. Present a scheme of classification of dyes based on their application.
7. Give examples of : (I) Triphenyl methane dye (ii) azodyes (iii) Anthraquinone dye.
8. What is a mordant dye ? How is it applied to the fabric ?
9. Bring out the essential point of difference between acidic dyes and basic dyes.
10. What are the essential components of a talcum powder ? What is the role of boric acid in talcum powder ?
11. What are deodorants and what is their specific role in cosmetics ?
12. What the essential components of a perfume ? How does the function of a perfume a perfume differ from cream ?
13. What are carbon fibres ? How they are designed ? Write two important uses of carbon fibres.
14. List various types of ceramics and their uses.
15. What are super conducting ceramics ? write some uses of superconductor ceramics.
16. Write a brief note on micro alloys.
17. Describe the following with suitable examples.
(i) Presevative (ii) Artificial sweetners (iii) Antioxidants (iv) Edible colours.
18. What are detergents ? Give their scheme of classification. Why are detergents preferred over soaps ?
19. What are biodegradable and nonbiogradable detergents ? What are the consequences of using latter clas of detergents ?
20. What are pheomones ? Why are pheromones said to be the action specific agents ?
21. What is a propellant ? How are various rocket propellants classified ?
22. What propellants have been used in PSLV – C4 rocket ?
23. Describe the following with examples :
(i) Double base propellants (ii) Biliquid propellant (iii) Monoliquid propellant (iv) Hybrid propellant.
24. Discuss the role of redox phenomenon in the the context of rocket propellants.
25. Which chemical is responsible for the antiseptic properties of dettol ?