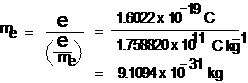UNIT 2 STRUCTURE OF ATOM
· Discovery of electron, proton and neutron.
· Atomic number, isotopes and isobars
· Thompson’s model and its limitations
· Rutherford ’s model and its limitations
· Bohr’s Model and its limitations
· Concept of shells and subshells
· Dual nature of matter and light
· De Broglie’s relationship
· Heisenberg’s uncertainty principle
· Concept of orbitals
· Quantum numbers
· Shapes of s, p, d orbitals.
· Rules for filling electrons in orbitals
· Aufbau principle
· Pauli’s exclusion principle
· Hund’s rule
· Electronic configurations of atoms
· Stability of half filled and completely filled orbitals.
SUBATOMIC AND FUNDAMENTAL PARTICLES
The existence of atoms has been proposed since the time of early Indian and Greek philosophers (400 B.C.) who were of view that atoms are the fundamental building blocks of matter i.e., the continued subdivision of matter would ultimately yield atoms which could not be further divisible. The word ‘atom’ has been derived from the Greek word ‘atmos’ which means ‘uncutable’ or ‘indivisible’.
The atomic model of matter was first proposed on a firm scientific basis by John Dalton, in 1808. His theory called ‘Dalton
Daton’s concept of indivisibiity of atom was disproved by experimental evidences obtained by scientists towards the end of nineteenth century. Researches done by various scientists like J.J.Thomson , Goldstein, Rutherford, Chadwick, Bohr and others have established that atom was not the smallest indivisible particle but had a complex structure of its own and was made up of still smaller particles like electrons, protons, neutrons etc. At present about 35 different subatomic particles are known but the three particles namely electron, proton and neutron are regarded as fundamental particles. The important properties of these fundamental particles are given in the Table.
Particle | Electron | Proton | Neutron |
Symbol | e | p | n |
Absolute charge C | -1.6022 x 10-19 | +1.6022 x 10-19 | 0 |
Relative charge | -1 | + 1 | 0 |
Mass kg | 9.10939 x 10-31 | 1.67262 x 10-27 | 1.67493 x 10-27 |
Mass u | 0.00054 | 1.00727 | 1.00867 |
Approximate mass u | 0 | 1 | 1 |
DISCHARGE TUBE EXPERIMENTS - DISCOVERY OF ELECTRONS
Under ordinary conditions gases are poor conductors of electricity. However, if a gas is taken in a sealed tube to which two electrodes are attached and if the gas pressure in the tube is reduced to about 10-2 atm, the gas becomes conducting on applying high voltage ( 5,000 - 10,000 volts ) to the electrodes. The gas was found to emit light under these conditions. The colour of the light depends upon the nature of the gas. The emission of light ceases as the pressure is reduced to 10-4 atm, but the gas continues to conduct electricity and the glass walls of the tube glows ( fluoresces)with faint greenish light . It was observed that an object placed inside the tube casts a sharp shadow on the walls of the glass tube. This experiment showed that the fluorescence was due to the bombardment of the glass by the rays emitted from the cathode and moving in straight lines. These rays are named as Cathode Rays . These rays were found to consist of negatively charged material particles called Electrons.
Fig. 1 . Generation of Cathode rays
The cathode rays possess the following properties:
1. Cathode rays travel in straight lines. An object in the path of cathode rays casts a sharp shadow ( Fig 2). It shows that the cathode rays travel in straight lines.
Fig 2. Cathode rays cast shadow of objects
placed in the path.
2. Heating effect : When cathode rays focused on a thin metal
foil, it gets heated up to incandescence.
3. Cathode rays consist of material particles. This was indicated by the fact that a light paddle wheel placed in the path of cathode rays starts rotating Fig 3.
Fig. 3 . Cathode rays rotate light paddle wheel.
4. Effect of electric field : When electric field is applied to a stream of cathode rays, they get deflected towards positive plate ( Fig 4 ) . It showed that cathode rays themselves are negatively charged.
Fig 4. Effect of electric field on cathode rays.
5. Effect of magnetic field : When magnetic field is applied, the cathode rays get deflected ( Fig 5). The direction of deflection again indicates that the cathode rays are negatively charged.
Fig 5 . Effect of magnetic field on cathode rays.
6. On striking against the walls of the discharge tube, cathode rays produce faint greenish fluorescence.
7. Cathode rays ionise the gas through which they pass.
8. Cathode rays can penetrate through thin metal foils.
9. Cathode rays can produce X-rays when they are made to fall on the metals such as tungsten, copper etc.
The above mentioned properties of cathode rays indicated that they consists of a fast moving stream of negatively charged material particles. These particles were named Electrons.
DETERMINATION OF CHARGE TO MASS
( e / m) OF ELECTRON
J.J Thomson measured the ratio of electrical charge (e) to mass of electron (me) by using cathode ray tube and applying electrical and magnetic field perpendicular to each other as well as to the path of electrons.
Thomson argued that the amount of deviation of the particles from their path in the presence of electrical or magnetic fields depends on :
(i) the magnitude of negative charge on the particle, greater the magnitude of the charge on the particle, greater is the interaction with electric or magnetic field and thus greater the deflection.
(ii) the mass of the particle - lighter the particles, greater the
deflection.
(iii) the strength of the electrical or magnetic field – the deflection of electrons from its original path increases with the increase in voltage across the electrodes , or strength of the magnetic field.
When only electric field is applied , the electrons deviate from their path and hit the cathode ray tube at point A. Similarly when only magnetic field is applied, the electron strikes the cathode ray tube at point C. By careful balancing the electric and magnetic field strength, it is possible to bring back the electron to the path followed as in the absence of electric and magnetic field and they hit the screen at point B. By carrying out accurate measurements on the amount of deflections observed by electrons on the electric field strength or magnetic field strength, Thomson was able to determine the value of e / em as e/me = 1.758820 x 1011C kg−1
where me is the mass of electron in kg and e is the magnitude of charge on the electron in coulomb (C). Since electrons are negatively charged, the charge on the electron is -e.
The e/me ratio for the particles in the cathode rays was found to be the same irrespective of the nature of the gas taken in the discharge tube, thus showing that the electrons are universal constituents of all matter.
THE CHARGE ON THE ELECTRON
The charge on the electron was determined by R.A. Milliken by using ‘oil drop’ experiment. In this method a spray of oil droplets is produced by an atomiser (Fig 7) .
The oil droplets enter the apparatus through a small hole and are allowed to fall between the charged plates. The motion of the droplets is observed with a telescope. The space between charged plates is irradiated with X-rays. The X-rays ionise the molecules of air. The oil droplets as a result becomes negatively charged. By measuring the velocity of a given droplet as it falls freely under the influence of gravity and then in an electric field, it is possible to calculate charge (q) on the droplet. Millikan found charge on all oil droplets which could be expressed as whole number multiple of ‘e’, which was considered to be the electronic charge. The charge on the electron is found to be 1.6022 x 10-19 coulomb.
The mass of electron varies with its speed. Since charge on the on the electron , e = - 1.6022 x 10-19 coulomb and at low speed, e/me = 1.758820 x 1011C kg−1
This is called rest mass of the electron, i.e., the mass which it possess when it is moving with the speed much smaller than that of light. The average mass of hydrogen atom is 1837 times greater than the mass of electron. The mass of electron on amu scale is 0.00054 .
ANODE RAYS
Goldstein (1886) discovered the existence of a new type of rays in the discharge tube. He used a perforated cathode (Fig 8 ) in the discharge tube.
Fig. 8. Origin of Anode rays.
On passing the electric discharge at low pressure , he observed a new type of rays streaming behind the cathode. These rays are named as anode rays or canal rays. Further investigations of these rays showed that they consist of positively charged material particles. Some of the characteristic properties of anode rays are :-
i) Anode rays are deflected by electric field towards negatively charged plate. This deflection indicates that they are positively charged.
ii) Anode rays are deflected by magnetic field. The direction of deflection indicates that they are positively charged.
iii) Charge to mass ratio of these particles in the canal rays depends upon the nature of the gas taken in the discharge tube.
Origin of Cathode rays and Anode rays
Under the influence of high potential difference, the gas in the discharge tube is ionised. This results in the formation of particles with positive and negative charge. The negatively charged particles ( electrons ) move towards the anode at very high speeds. On the their way they collide with the molecules of the gas producing more electrons and positively charged particles. The electrons move towards anode in the form of cathode rays while positive ions move towards cathode in the form of anode rays or canal rays.
Discovery of Proton
The charge to mass ratio of the particles in the anode rays was found to depend upon the nature of the gas in the discharge tube. It was observed that e/m ratio was maximum when hydrogen was taken in the discharge tube. This indicated that the positive ions formed from hydrogen are lightest. These lightest positively charged particle was named proton. The charge to mass ratio was determined and the value was found to be 9.58 x 107 C kg-1. The charge on the proton is opposite but equal in magnitude to the charge on the electron, i.e., 1.6022 x 10-19 Coulomb. From these observations the mass of a proton works out to be 1.67262 x 10-27 kg. This is nearly 1836 times that of electron or nearly 1.00727 on atomic mass scale i.e., practically equal to that of hydrogen atom. This lightest positive particle is known as proton. The hydrogen atom which is the smallest atom known, consists of only one proton and one electron.
The existence of charges on cathode and anode rays indicates that the atom consists of one or more electrons and a positive residue. Since electrons have a negligible mass, it follows that almost the entire mass of an atom is associated with the positive residue.
RADIOACTIVITY
The discovery of the cathode and anode rays showed that the atoms are divisible into sub-atomic particles. This was further supported by the phenomenon of radioactivity discovered by Henry Bequerel (1896). The spontaneous emission of radiation by certain elements like Uranium is called radioactivity and the elements are called radioactive elements. On placing a sample of uranium mineral in a lead block and allowing the emitted rays to pass through electric and magnetic fields, the radiation is resolved into three directions ( Fig 9 ).
Fig 9. Radioactive rays.
a) The rays which are deflected slightly towards negative plate were named as a-rays.
b) The rays which are deflected towards positive plate were named b-rays.
c) The rays which remained undeflected were named as g-rays. a-rays consists of positively charged He2+ particles. The charge on a-particle is 3.20 x 10-19C and its mass is 6.6 x 10-27 kg. b-rays are made up of electrons , while g-radiation having no charge and negligible mass.
Discovery of Neutron
Neutrons were discovered by James Chadwick by the bombarding a thin film of Beryllium with fast moving alpha-particles. A highly penetrating rays consisting of neutral particles were produced. The emission of these particles were unaffected by electric and magnetic fields. These particles were called neutrons and their origin was explained on the basis of the following reaction.
The formation of isotopes with the same atomic number and different mass numbers were explained on the basis , that nuclei of different isotopes possess the same number of protons but different number of neutrons.
The mass of neutron was found to be 1.6748 x 10-27 kg which is 1839 times heavier than the electron.
A neutron is a sub-atomic particle carrying no charge and having mass 1.6748 x 10-27 kg which is almost equal to that of a hydrogen atom. The mass of the atom is largely due to the protons and neutrons in the nucleus of the atom.